Abstract
Introduction: Systemic immunoglobulin light chain (AL) amyloidosis features the production of unstable light chains (LCs) by malignant plasma cells in the bone marrow. Unstable LCs misfold into amyloid fibrils that deposit in various organs. The heart is the most commonly affected organ. Early mortality and poor tolerance to therapy remain significant challenges in clinical management, particularly in patients with advanced cardiac involvement. A growing body of evidence suggests that LC reduction from plasma cell directed therapy can lead to improved cardiac function despite persistent architectural distortion from amyloid deposits. However, there is a limited understanding of the underlying molecular mechanisms of LC-induced cardiotoxicity. We evaluated transcriptomic changes in cardiac cells upon exposure to cardiotropic, amyloidogenic LCs. We sought to characterize the earliest signs of LC-induced cardiotoxicity and to validate our findings using induced pluripotent stem cell (iPSC)-derived cardiomyocytes.
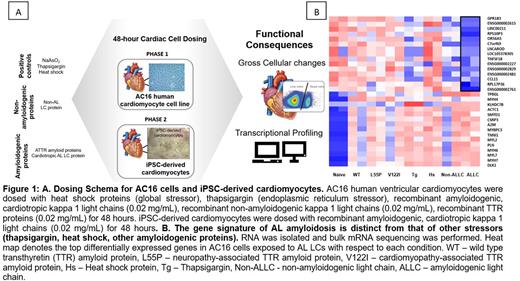
Methods: LC proteins were prepared from a patient with severe cardiac AL amyloidosis and an associated clonal plasma cell disorder. AC16 human ventricular cardiomyocytes as well as iPSC-derived cardiomyocytes were exposed to LCs for 48 hours and assayed for gross morphological changes via light microscopy. We also performed flow cytometry to evaluate cell viability and cytotoxicity. Gene expression changes were assessed using mRNA sequencing with RT-PCR for hit validation. Functional changes were also assessed in iPSC-derived cardiomyocytes following LC exposure. Heat shock (42°C for 110 minutes), thapsigargin (an endoplasmic reticulum stressor and activator of the unfolded protein response), and transthyretin amyloid (ATTR) proteins were alternate stressors included as controls to aid in the identification of LC-specific transcriptional signatures. (Figure 1A)
Results: AC16 cells exhibited predictable responses to the stressors included as controls, specifically upregulation of heat shock response genes (HSPA6, HSPA7, and HSPA1B) and genes associated with the UPR (XBP1, ATF6, and PERK) upon exposure to heat shock and global ER stressor thapsigargin, respectively. AC16 cells exposed to cardiotropic, amyloidogenic LCs displayed a higher number of significantly differentially expressed genes (7241) as compared to TTR proteins (TTRWT 4103, TTRV122I 2365, and TTRL55P 396) highlighting the complex response of cardiac cells to cardiotropic, amyloidogenic LCs. There was no overlap in the gene expression changes in AC16 cells exposed to AL LCs versus ATTR suggesting there is unique gene signature associated with LC-induced cardiotoxicity. (Figure 1B) By RNASeq, we identified gene expression changes in hallmark pathways related to cardiac contractility (XIRP1, PFKFB1), muscle development (actin, myosin, and troponin genes), cardiac remodeling (proteoglycan, glycosaminoglycan, metalloproteinases, and tissue inhibitors of metalloproteinase genes), and adaptive immune response (GPR183, IL1RL1). Our findings were confirmed via RT-PCR analysis of select target genes that were significantly differentially regulated or involved in pathways regulated by known therapeutics. Independently, iPSC-derived cardiomyocytes were employed to further validate our findings. Gross assessment of cardiac chronotropy revealed that cardiotropic, amyloidogenic light chains induce negative chronotropic effects on iPSC-derived cardiomyocytes within 24 hours of exposure.
Conclusion: Here, we reveal the possible molecular events occurring in cardiomyocytes after exposure to cardiotropic, amyloidogenic LCs and prior to marked cardiomyocyte damage. Upregulation of transcripts related to cardiac contractility and muscle development, may provide evidence for the development of an adaptive hypertrophic phenotype that reduces oxygen consumption, diminishes cardiomyocyte stress, and preserves cardiac function in the initial phases of LC exposure. Our findings could support a rationale for developing biomarkers and novel therapeutics. Moreover, our iPSC-based system could provide a platform for identifying patients with premalignant and malignant plasma cell disorders at risk of organ damage from light chains.
Disclosures
Sanchorawala:Proclara, Caelum, Abbvie, Janssen, Regeneron, Protego, Pharmatrace, Telix, Prothena: Consultancy; Celgene, Millennium-Takeda, Janssen, Prothena, Sorrento, Karyopharm, Oncopeptide, Caelum, Alexion, Pfizer, Janssen, Attralus, Proclara, Caelum, Abbvie, Janssen, Regeneron, Protego, Pharmatrace, Telix, Prothena: Consultancy, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal